Catalytic Converters Reduce Pollution
A catalyst is a substance that causes or accelerates a chemical reaction without itself being affected. Catalysts participate in the reactions, but are neither reactants nor products of the reaction they catalyze.
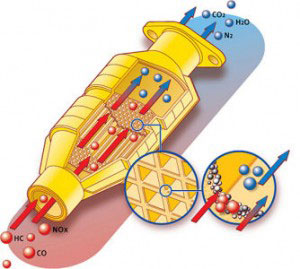
There are two different types of catalysts in a catalytic converter. A reduction catalyst and an oxidation catalyst. Both consist of a ceramic structure coated with a metal catalyst, usually platinum, rhodium and/or palladium. This creates a structure that exposes the maximum surface area of catalyst to the exhaust stream, whilst minimizing the amount of catalyst required. Some of the new converters have started to use gold mixed with the traditional catalysts which could increase oxidation.
Most modern cars are equipped with three-way catalytic converters. This refers to the three regulated emissions it helps to reduce.
The reduction catalyst is the first stage of the catalytic converter. It uses platinum and rhodium to help reduce the NOx emissions. When an NO or NO2 molecule contacts the catalyst, the catalyst rips the nitrogen atom out of the molecule and holds on to it, freeing the oxygen in the form of O2. The nitrogen atoms bond with other nitrogen atoms that are also stuck to the catalyst, forming N2. For example:
2NO => N2 + O2 or 2NO2 => N2 + 2O2
Ceramic honeycomb catalyst structure.The oxidation catalyst is the second stage of the catalytic converter. It reduces the unburned hydrocarbons and carbon monoxide by burning (oxidizing) them over a platinum and palladium catalyst. This catalyst aids the reaction of the CO and hydrocarbons with the remaining oxygen in the exhaust gas. For example:
2CO + O2 => 2CO2
There are two main types of structures used in catalytic converters — honeycomb and ceramic beads. Most cars today use a honeycomb structure.
Inside the cat!